DOI: 10.1002/anie.202423092
文章摘要
NH4H2PO4和KH2PO4复合肥料在提高作物产量和品质方面具有显著优势。氨(NH₃)对于合成这种复合肥是必不可少的,它是通过一个能源密集型的化学过程生产的。开发高效、环保的方法生产含氨复合肥至关重要,而且极具挑战性。在这里,我们提出了一个串联催化系统,能够通过电化学和后续化学过程相结合,在工业规模的反应器中将大量尿素完全转化为复合肥。在一个典型循环中,使用200 g尿素,无需进一步分离,可产生1580 g固体复合肥(KH2PO4和NH4H2PO4)和232 L纯H2。实现该系统的关键是精确控制电化学过程中尿素的消耗速率和NO2-的生成速率。通过在电解液中保持尿素与NO2-的精确摩尔浓度比为1/2,尿素可以与NO2-完全反应生成N2,而CNO-可以在H3PO4处理的第二步中转化为NH4+生成复合肥,实现尿素的完全转化而不产生副产物。
图文导读
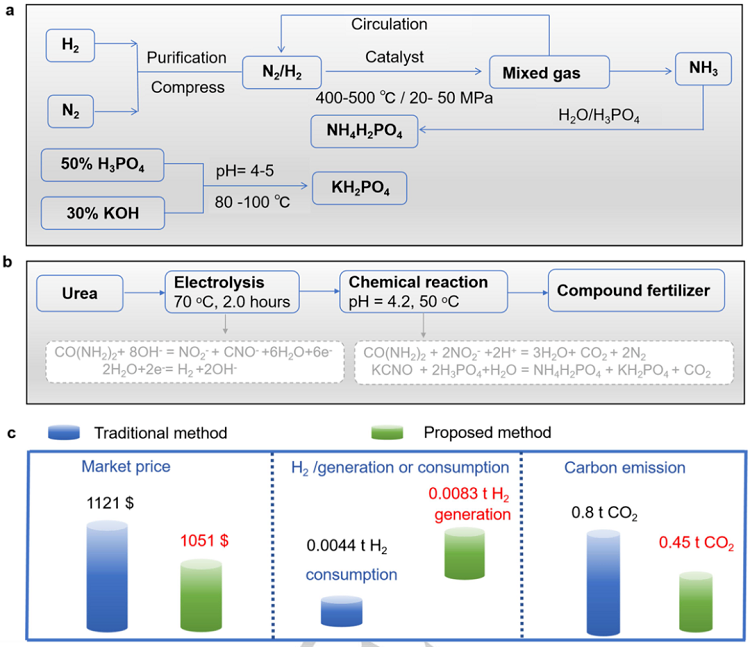
Figure 1. Comparison of the proposed method and traditional method. (a) Schematic illustration of the traditional method for production of the compound fertilizer.(b) Schematic illustration of the tandem system for production of the compound fertilizer. (c) Comparison of the proposed method and traditional method regardinganticipated market price, H2 generation/consumption, and carbon emissions per ton of compound fertilizer.
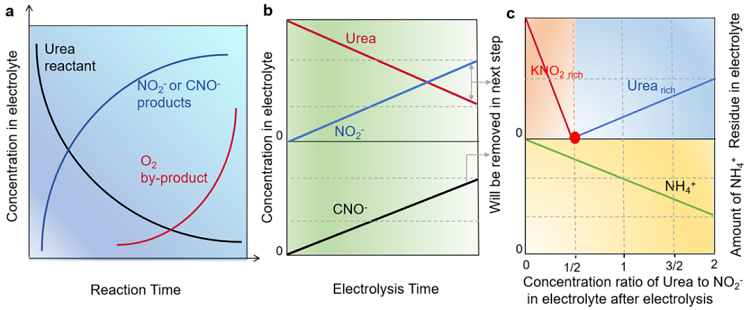
Figure 2. Theoretical advantages of the tandem industrial-scale system. (a) A diagram of the relationship between urea, NO2-, CNO- and O2 concentrations andelectrolysis time during one cycle. (b) A simplied diagram illustrating the relationship between the concentration of urea, NO2- and CNO- and the electrolysis timein the electrolytic process. (c) A diagram illustrating the correlation between the residual amount (urea and KNO2), as well as NH4+ levels after acid treatment, inrelation to the concentration ratio of urea to NO2- in the electrolyte.
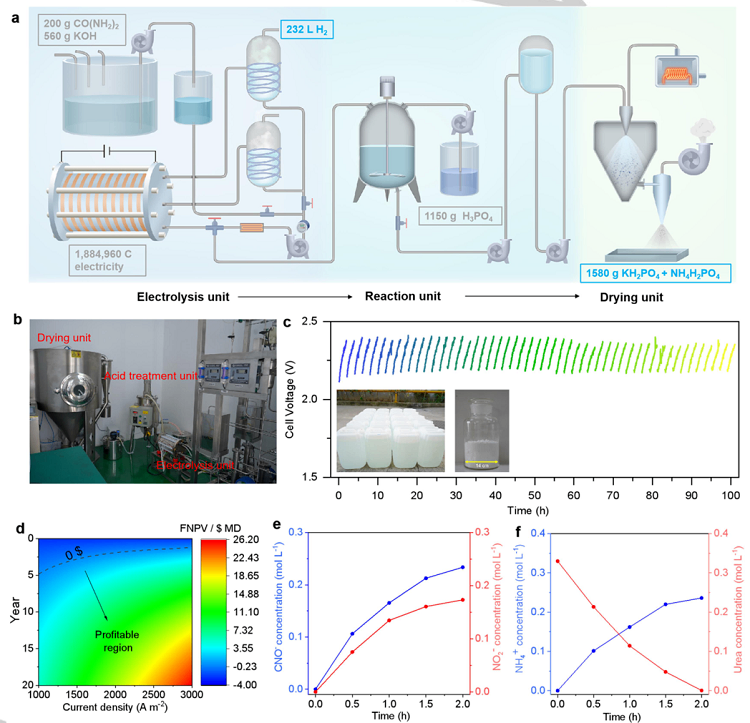
Figure 3. Implementation of the tandem industrial-scale system. (a) An implementation flowchart. (b) Photographs of the electrolysis unit, reaction unit (acidtreatment unit) and a drying unit within the tandem system. (c) Stability evaluation of the electrochemical process at a constant current density of 200ௗmAௗcm-2 at70ௗ°C. (inset, Photographs of the electrolyte after 50 electrolysis cycles and solid fertilizer in a cycle). (d) Economic profitability diagram. (e) Concentrations ofNO2- and CNO- during UOR as a function of electrolysis time. (f) Concentrations of urea and NH4+ after the tandem system as a function of electrolysis time.
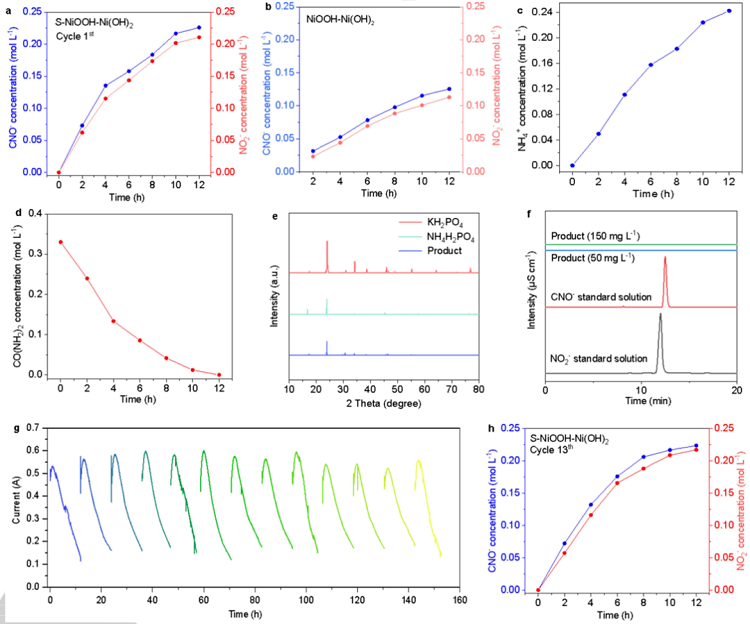
Figure 4. In-depth verification of the tandem industrial-scale system. (a) Concentrations of NO2- and CNO- in the electrolyte solution during UOR (driven by S-NiOOH-Ni(OH)2) as a function of time under the voltage of 0.52 V vs Hg/HgO. (b) Concentrations of NO2- and CNO- in the electrolyte solution during UOR (drivenby NiOOH-Ni(OH)2) as a function of time under the voltage of 0.52 V vs Hg/HgO. (c-d) Concentration of NH4+ and urea in the solution after the urea oxidation andtreatment with H3PO4. (e) XRD patterns of the product (with only KH2PO4 and NH4H2PO4), and KH2PO4 and NH4H2PO4 standard sample. (f) Results of ionchromatography for product with only KH2PO4 and NH4H2PO4 compared with standard anion solutions. (g) I-t curve for 13 cycles. (h) Concentrations of NO2- andCNO- in the electrolyte solution during UOR after 13 cycles.
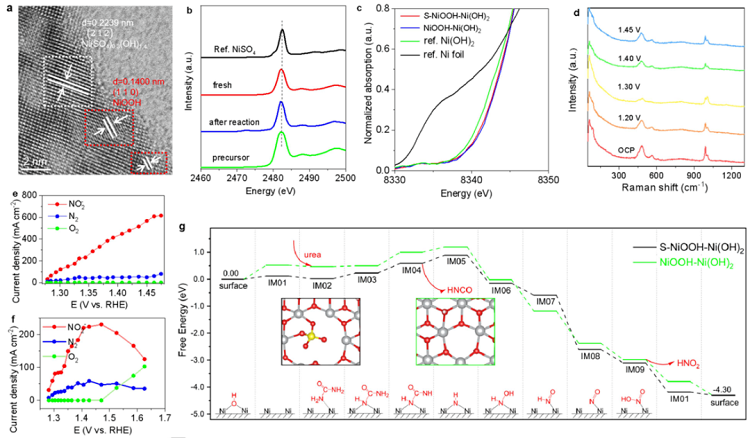
Figure 5. Active ingredient of typical S-NiOOH-Ni(OH)2 catalyst, UOR performance in a three-electrode cell and DFT computations. (a) TEM image. (b)Synchrotron radiation near side X-ray absorption fine structure (NEXAFS) spectroscopy at the S K-edge for different samples. (c) Normalized Ni K-edgeXANES spectra of Ni foil, Ni(OH)2, NiOOH-Ni(OH)2 and S-NiOOH-Ni(OH)2. (d) In-situ Raman spectra in 1.0 mol L−1 KOH with 0.33 mol L−1 urea solution atvarious potentials for S-NiOOH-Ni(OH)2. (e) Current density of NO2-, N2 and O2 for typical S-NiOOH-Ni(OH)2 at different potential. (f) Current density of NO2-,N2 and O2 for NiOOH-Ni(OH)2 at different potentials. (g) The chemisorption of urea molecule on constructed double-layer catalytic models (S-NiOOH-Ni(OH)2)and the control group (NiOOH-Ni(OH)2), with skeletal formula representing the corresponding intermediate structure. Ordinates represent the Gibbs free energyfor each intermediate under the experimental condition (i.e. urea 0.33 mol·L-1, OH- 1.00 mol·L-1, working voltage -1.28 V versus RHE). The structure charts withblack and green borders respectively represent the IM01 on S-NiOOH-Ni(OH)2 and NiOOH-Ni(OH)2. Legend: silver, Ni; red, O; white, H; blue, N; black, C. Inset,The elementary reaction channel for OER at open circuit potential, with the arrow indicating the elementary reaction requiring the highest working voltage.
总结与展望
我们提出了一种新的解决方案,以现实的工业催化过程为例(阳极电催化尿素氧化与阴极氢气生产耦合)。提出了一种将电化学过程和后续化学过程相结合的串联系统,该系统不仅可以消除尿素,还可以生产氢气和纯肥料,实现尿素资源的充分利用。该系统执行工业级工艺(每个循环使用10个电解液),将尿素(或尿素废水)完全转化为有价值的化学品(纯复合肥料,价格低于当前市场)。在一个典型的循环中,生产1580克复合肥料和232升氢气,而生产复合肥料的传统方法需要消耗氢气。该系统有可能使用部分清洁能源来实现低碳目标。在该体系中,通过一种简单、易于扩展的方法,开发出了NO2-和CNO-产率高、稳定性好的高性能UOR电催化剂。
材料合成
NiOOH@Ni(SO4)0.3(OH)1.4(记为S-NiOOH-Ni(OH)2)。首先,通过水热反应在泡沫镍上合成了典型的S-NiOOH-Ni(OH)2。通常,2×3 cm2的泡沫Ni在1.0M HNO3溶液、乙醇和去离子水中通过超声彻底清洗。随后,将1.16 g六水硝酸镍和200 mg过硫酸钾溶解于30 mL去离子水中。将得到的均质溶液转移到内衬聚四氟乙烯的不锈钢高压灭菌器中,将清洁的泡沫Ni(2×3 cm2)浸入其中,在150℃下保持10 h。自然冷却至室温后,收集样品,用乙醇反复洗涤,然后在80℃烘箱中干燥12 h,得到Ni(OH)2@Ni(SO4)0.3(OH)1.4。然后用次氯酸钠溶液处理样品,得到最终产物S-NiOOH-Ni(OH)2。在次氯酸钠溶液中溶解1.6 g氢氧化钠(有效氯含量6.0%)。随后,将Ni(OH)2@Ni(SO4)0.3(OH)1.4浸入反应溶液中,在35℃下保持3 h。收集所得产物,用去离子水冲洗多次,在60℃烤箱中干燥24 h,得到最终的S-NiOOH-Ni(OH)2。
NiOOH@Ni(OH)2(记为NiOOH-Ni(OH)2)。通常,将1.5 mmol六水硝酸镍和2 mmol尿素溶解在30 mL去离子水中。将2×3 cm2的泡沫Ni浸入反应溶液中,将其转移到50 mL反应釜中,在200℃下保持12 h。冷却至室温后,收集样品,用蒸馏水洗涤数次,在120℃烤箱中干燥24 h,生成Ni(OH)2。然后用次氯酸钠溶液处理Ni(OH)2,得到最终产物NiOOH-Ni(OH)2。过程类似于NiOOH@Ni(SO4)0.3(OH)1.4。通常将1.6 g氢氧化钠溶解在次氯酸钠溶液中(有效氯为6.0%)。将Ni(OH)2片浸入反应溶液中,在35℃下保持3 h,收集产物,用去离子水冲洗数次,在60℃烤箱中干燥24 h,得到NiOOH@Ni(OH)2。
本文实验中使用的原位红外电化学ATR系统为合肥原位科技有限公司研发。感谢老师支持和认可!
